At presynaptic terminals, synaptic vesicle membranes are fused into plasma membrane upon exocytosis. After exocytosis, fused vesicle membranes are retrieved by endocytosis into presynaptic terminals to be reused. During sustained high frequency transmission, exo-endocytic coupling is critical for the maintenance of synaptic transmission. Here we show that this homeostatic coupling is supported by cGMP-dependent protein kinase (PKG) at the calyx of Held. Pharmacological tests with capacitance measurements revealed that presynaptic PKG activity accelerates vesicle endocytosis, and is supported by a retrograde signal cascade mediated by NO that is released by activation of postsynaptic NMDA receptors by the neurotransmitter glutamate. Activation of PKG also up-regulates phosphatidylinositol-4,5-bisphospate (PIP2), thereby accelerating endocytosis. By accelerating vesicle endocytosis, presynaptic PKG activity supports synaptic fidelity during high frequency transmission. These mechanisms start to operate after hearing onset during the second postnatal week, when PKG expression becomes upregulated in the brainstem. We conclude that maturation of the PKG-dependent retrograde signal cascade strengthen the homeostatic plasticity for the maintenance of high frequency synaptic transmission at the calyx of Held synapse.
(Eguchi K, Nakanishi S, Takagi H, Taoufiq Z, Takahashi T. Neuron; 74: 517-29, 2012.)
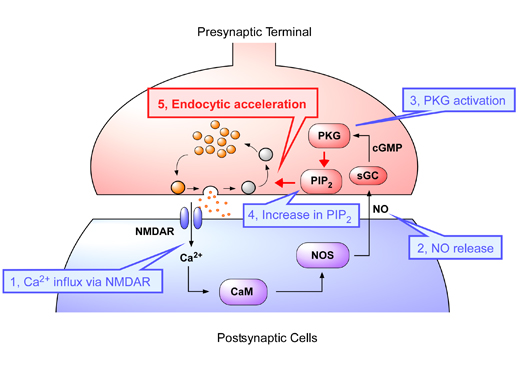
Figure: Retrograde regulation of vesicle endocytosis by nitric oxide
After the neurotransmitter glutamate is released from the presynaptic terminal, it binds to postsynaptic NMDA receptors and induces Ca2+ influx into the postsynaptic cell. The high calcium concentration may activates calmodulin (CaM), thereby promoting NO synthesis by activating NO synthases (NOS). NO is diffused into presynaptic membranes and activates soluble guanylate cyclase (sGC), which synthesizes cGMP from GTP. Increasing cGMP activates cGMP-dependent protein kinase (PKG) and PKG regulates PIP2 concentration on terminal membranes, thereby accelerating vesicle endocytosis. The extent of this feedback regulation depends upon the amount of glutamate released. Thus, the NO-PKG-PIP2 retrograde pathway couples exo-endocytosis of synaptic vesicles for the maintenance of long-lasting synaptic transmission.
*Cellular & Molecular Synaptic Function Unit, Okinawa Institute of Science and Technology Graduate University