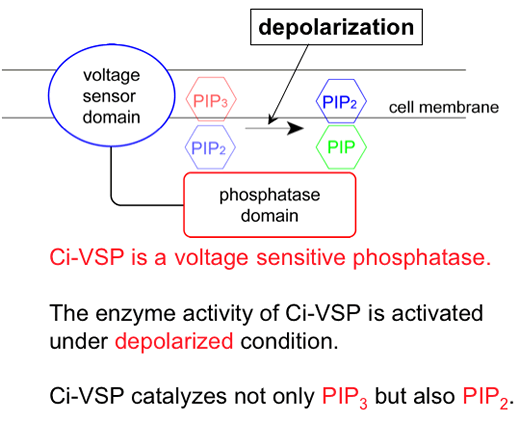
We previously reported an ascidian protein Ci-VSP which has a transmembrane voltage sensor motif and a phosphatase domain. We showed that the voltage sensor domain functionally couples with the phosphatase domain and the phosphatase activity increases by depolarization. However, it remained unknown whether depolarization or hyperpolarization induced activation of enzyme. In order to address this issue, we performed (1) measurements of phosphoinositide level using GFP-based imaging by confocal microscopy under voltage clamp condition in Xenopus oocyte and (2) detailed electrophysiological analysis of modification of three types of potassium channels by Ci-VSP.
PtdIns(4,5)P2 level, as detected by PH(PLC-δ)-GFP, decreased by depolarization and increased by hyperpolarization, consistent with our previous report (Murata et al, 2005). However, PtdIns(3,4,5)P3 level as detected by PH(Btk)-GFP also showed similar change as PtdIns(4,5)P2, as opposed to the idea that Ci-VSP dephosphorylates PtdIns(3,4,5)P3 to increase PtdIns(4,5)P2.
Next, the activities of IRK1, with higher affinity to PtdIns(4,5)P2 than GIRK2 were measured: it showed current decrease dependent on the membrane potential of intervals with the rightward shift of the current-voltage relationship compared with that of GIRK2 channels. In addition, we noticed that KCNQ2/3 channels in the presence of Ci-VSP showed remarkable current decay similar to channel inactivation at the higher membrane potential. According to depolarization the decay time constant became gradually smaller, indicating that phosphatase activity increases up to about 100 mV.
Taken together, we conclude that (1) phosphatase activity of Ci-VSP turns on by depolarization and (2) PtdIns(4,5)P2 as well as PtdIns(3,4,5)P3 can be a substrate for Ci-VSP.
Murata Y. and Okamura Y., J. Physiol., 583: 875-889(2007)
* Section of Developmental Neurophysiol., Okazaki Institute for Integrative Biosci., NINS, Aichi, Japan,
Present address: Department of Physiology I, Tohoku University Graduate School of Medicine, Miyagi, Japan