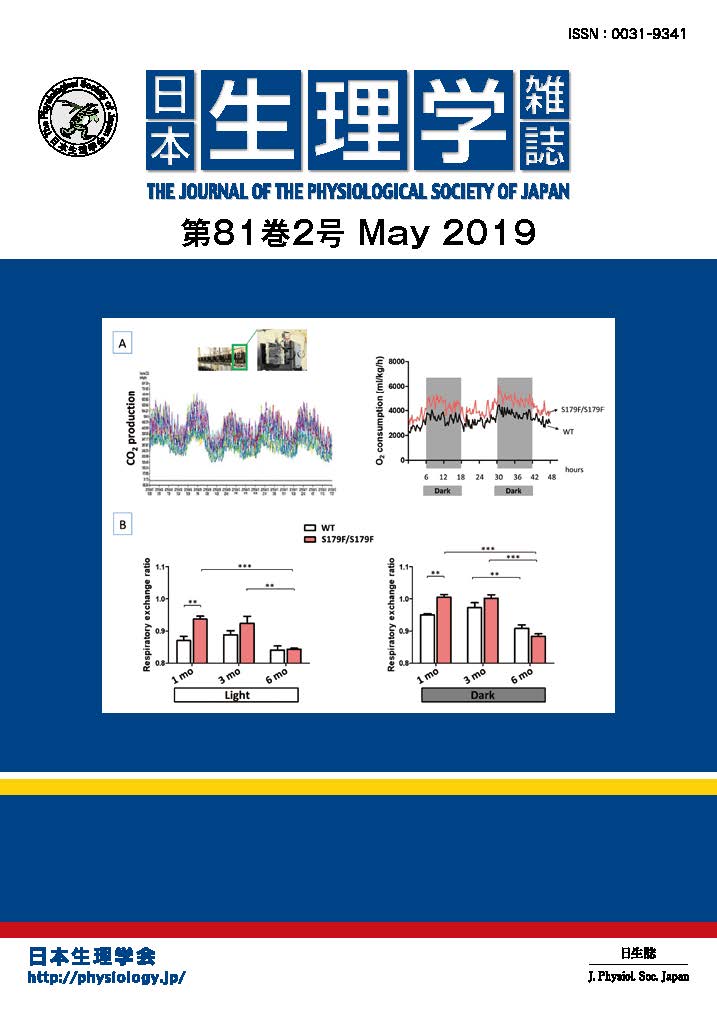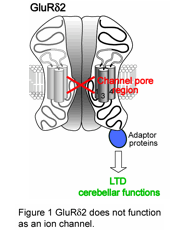
The δ2 glutamate receptor (GluRδ2), which is predominantly expressed in cerebellar Purkinje cells, is a member of ionotropic glutamate receptor (iGluR) family. The GluRδ2 plays crucial roles in synapse formation and synaptic plasticity: mice disrupted GluRδ2 gene (GluRδ2-null mice) showed severe ataxia, abnormal synapse morphology and impaired cerebellar long-term depression (LTD), a synaptic plasticity model responsible for motor learning. Despite its importance, the
mechanisms by which GluRδ2 participates in cerebellar functions, especially whether GluRδ2 functions as a channel, is a long-lasting question because of the lack of ligands for GluRδ2. To address this issue, we introduced two kinds of mutant GluRδ2s, in which putative sites underlying (1) Ca2+-permeability and (2) channel pore are disrupted, into GluRδ2-null cerebellum. Surprisingly, transgenic mouse-mediated expression of mutant GluRδ2 lacking Ca2+-permeability rescued almost all abnormality of GluRδ2-null mice (Kakegawa et al., J. Physiol., 579: 729-735, 2007). Furthermore, Sindbis virus-mediated expression of mutant GluRδ2 disrupted the putative channel pore also recovered impaired LTD of GluRδ2-null Purkinje cells (Kakegawa et al., J. Physiol., 584: 89-96, 2007). These results strongly supported that, although GluRδ2 belongs to iGluRs, GluRδ2 does not serve as an ion channel in vivo (Figure 1). Accumulating evidence indicated that other iGluRs also have non-ionotropic functions, therefore, GluRδ2 may provide a key insight into the elucidation of non-ionotropic functions of iGluRs.
*Department of Physiology, Keio University School of Medicine