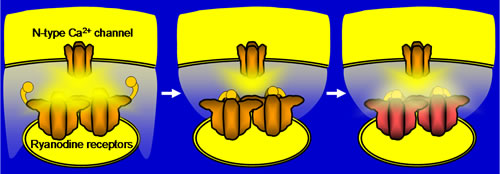
Ca2+-induced Ca2+ release (CICR) via ryanodine receptors (RyRs) in the somata of bullfrog sympathetic neurons plays a very important role in regulating membrane excitability (Akita & Kuba, J Gen Physiol 116:697-720, 2000). In our recent study, we found that this CICR is tightly regulated by a Ca2+-dependent “inactivation” mechanism (Akita & Kuba,J Physiol 586:3365-84, 2008). The observable [Ca2+]i rise evoked by Ca2+ entry at the beginning of membrane depolarization was solely due to CICR in this neuron, and this CICR was inactivated within 10-20 ms when the Ca2+ entry continued. The inactivation was inhibited by intracellular BAPTA (IC50»0.4 mM) but not by EGTA (≦10 mM), indicating that it must be mediated by some Ca2+-sensing molecules located close to (at ~60 nm from) voltage-gated Ca2+ channels and/or RyRs, and that the molecules must be exposed to a high [Ca2+]i at the edges of “Ca2+ microdomains” during Ca2+ entry. Moreover, the longer duration of Ca2+ entry persisting after CICR inactivation was found to cause slower [Ca2+]i decay after the end of Ca2+ entry. This was inhibited in parallel with the inhibition of inactivation by BAPTA. Thus, some mechanism counteracting Ca2+ clearance must be linked to the inactivation mechanism, and this should provide the basis for the prolonged suppression of membrane excitability through activation of Ca2+-sensitive K+ channels after a longer period of membrane depolarization.
Supplementary information for figure: The Ca2+ sensor for inactivation is highly likely to reside in the molecules different from RyRs, although they are yet to be identified. The mechanism counteracting [Ca2+]idecay would presumably be the weak Ca2+ release from RyRs in the “flickeringly” open mode, which must be converted from the inactivated state.
*Laboratory of Correlative Physiology, National Institute for Physiological Sciences